What is VIA Disc NP?
VIA Disc NP is intended for use as an allograft to supplement degenerated intervertebral discs. An allograft is tissue recovered from a human cadaveric donor that is transferred to a human recipient. VIA Disc NP consists of dehydrated nucleus pulposus particulate derived from the intervertebral disc region of the donor. The nucleus pulposus particulate is mixed together with saline and delivered into your intervertebral disc during a non-surgical spinal procedure.
How Does VIA Disc NP Work?
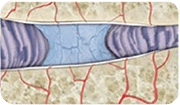
Age-related wear and tear of the intervertebral disc can cause loss of hydration and degeneration.

VIA Disc NP is delivered into the degenerated intervertebral disc through a 22G spine needle.
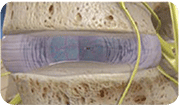
After delivery, VIA Disc NP supplements the degenerated intervertebral disc.
How can intervertebral discs degenerate?
Intervertebral discs can deteriorate through normal aging and injury, causing dehydration, flattening, and loss of natural cushioning. Just like grapes lose water over time and turn into raisins, your intervertebral discs undergo a similar dehydration process. This process leaves your discs vulnerable to motion stress, strains your spinal nerves, and can result in lower back pain.
What are the Indicators of Degenerated Intervertebral Discs?
- Degenerative Disk Disease
- Discogenic Pain
WHAT ARE THE VIA DISC NP KEY FEATURES?
Non-Surgical
- VIA Disc NP is a non-surgical option that can be delivered through a 22G spin needle.
Ease of Use
- Off-the-shelf allogeneic graft for ease of use.
Proprietary System for Allograft Preparation
- Consistent mixing in a fully closed system to reduce contamination risk.
What to Expect with VIA Disc NP?
Pre-Procedure
The nucleus pulposus particulate is mixed together with saline for delivery into your intervertebral disc.
Intravenous antibiotics may be given before the VIA Disc NP Procedure to reduce risk of infection.
Procedure
The VIA Disc NP procedure is performed under strict sterile conditions.
The VIA Disc NP procedure can be performed under local anesthesia or moderate sedation may be recommended by your physician. A procedure involving your pain-generating intervertebral disc can be painful.
During the procedure, your physician will use fluoroscopy (a computer tomography scan that continues to take images during the procedure so that the physician can see where the needle is going).
Your physician will insert a needle through the skin and muscle into the center of the intervertebral disc. VIA Disc NP will slowly be delivered into the center of the intervertebral disc.
Post-Procedure
You may experience pain and soreness after the procedure, which is normal. This pain may be due to increased pressure within your intervertebral disc.
It is important to follow your physician’s instructions after the procedure. You may be instructed to keep your activity to the normal activities of daily life and limit physical or strenuous activity for 72 hours post-procedure.
Your physician may prescribe certain pain medications (e.g. analgesics, steroid dose pack, muscle relaxants). You may experience moderate to severe pain after an injection into an intervertebral disc, and oral medications may be needed promptly to treat this post injection pain and discomfort. An ice pack may be given to place over the injection site in the event of post-injection site discomfort. Your physician may suggest a back brace or recommend physical therapy to make you feel more comfortable following your procedure.
A follow-up appointment will typically be scheduled two to four weeks after the procedure to monitor your pain and comfort. Additional follow-up appointments may be scheduled at the discretion of your physician to assess your condition.
It is important that you consult with your physician for medical advice, including any questions you may have regarding the VIA Disc NP product or the associated procedure.
VIA Disc NP Allograft
This information is designed to inform you about the VIA Disc NP allograft product and procedure. It is not meant to replace personal conversations with your physician or other members of your healthcare team. This information is intended to answer some of your questions and serve as a guide for you to ask appropriate questions about the product and the procedure.
Risks with Allograft Products like VIA Disc NP and the Associated Procedure
Careful donor screening, laboratory testing, and tissue processing, including sterilization via electron-beam irradiation of the disc tissue, have been used to minimize the risk of transmission of applicable diseases to the patient. Tissue donors are thoroughly screened and tested to meet or exceed safety standards mandated by the FDA and AATB. As with any processed human donor tissue, VIA Disc NP cannot be guaranteed to be free of all pathogens. CONTRAINDICATIONS: VIA Disc NP is contraindicated in patients with known sensitivities to Gentamicin, Vancomycin, or Bacitracin. ADVERSE EVENTS: Possible adverse events may include: Transmission of disease of unknown cause and transmission of infectious agents including but not limited to: HIV, hepatitis, syphilis, or microbial contaminants, pain and/or inflammation/swelling near the injection area in your spine or back, hematoma – a collection of blood at the site of the injection, epidural bleedings – a collection of blood in the potential space between the dura (covering of the spinal cord) and the bone, along the spinal canal (hollow passage through the back bones through which the spinal cord runs), infections (for example, at the injection site, in the spinal disc or bone in your spine and/or meningitis), neurological deterioration, such as loss of feeling or tingling or weakness, as serious as paralysis of the legs or lower body, sexual dysfunction, cerebrospinal fluid fistula (CSF), a spinal fluid leak, relapsing herniation, herniated disc material at the same level as the procedure, bladder (urination) or bowel dysfunction, vertebral end plate inflammation, or damage to endplates can occur with disc and/or endplate degeneration.
